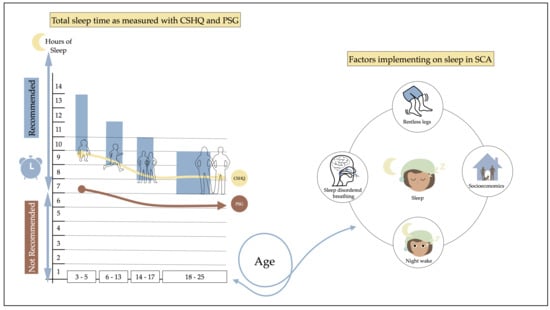
Developmental Profile of Sleep and Its Potential Impact on Daytime Functioning from Childhood to Adulthood in Sickle Cell Anaemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Selection and Description
2.2. Procedures
2.2.1. Polysomnography
2.2.2. Sleep Characteristics and Demographics Questionnaire
2.3. Statistical Analysis
3. Results
3.1. Recruitment and Baseline Characteristics
3.2. Comparison of Sleep Profiles as Measured with PSG and CSHQ
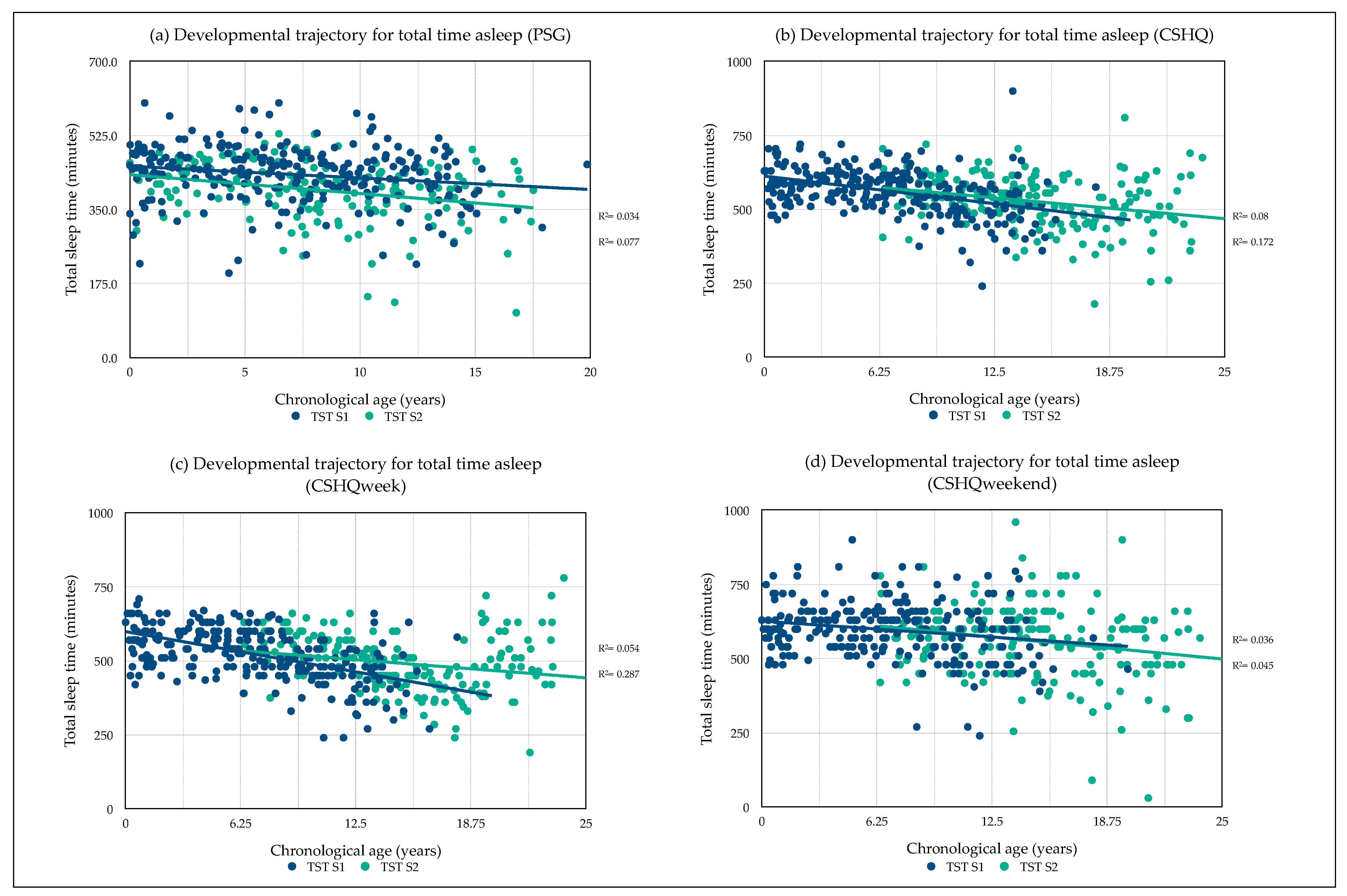
3.3. Developmental Sleep Profile Changes
3.4. Midpoint of Sleep
3.5. PSG: Clinical Sleep Characteristics
3.6. CSHQ: General Sleep Characteristics
3.7. Daytime Functioning
3.8. Predictors of Total Sleep Time
3.8.1. Predictors of Total Sleep Time (PSG)
3.8.2. Predictors of Total Sleep Time (CSHQ)
4. Discussion
4.1. Sleep Profiles: Differences between Polysomnography and Parent Reports
4.2. Sleep Characteristics
4.3. Daytime Functioning
4.4. Predictors of Total Sleep Time
4.5. Limitations/Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mascarenhas, M.I.; Loureiro, H.C.; Ferreira, T.; Dias, A. Sleep pathology characterization in sickle cell disease: Case-control study. Pediatr. Pulmonol. 2015, 50, 396–401. [Google Scholar] [CrossRef]
- Cahill, L.S.; Gazdzinski, L.M.; Tsui, A.K.; Zhou, Y.-Q.; Portnoy, S.; Liu, E.; Mazer, C.D.; Hare, G.M.; Kassner, A.; Sled, J.G. Functional and anatomical evidence of cerebral tissue hypoxia in young sickle cell anemia mice. J. Cereb. Blood Flow Metab. 2017, 37, 994–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawadler, J.M.; Kirkham, F.J.; Clayden, J.D.; Hollocks, M.J.; Seymour, E.L.; Edey, R.; Telfer, P.; Robins, A.; Wilkey, O.; Barker, S.; et al. White Matter Damage Relates to Oxygen Saturation in Children With Sickle Cell Anemia Without Silent Cerebral Infarcts. Stroke 2015, 46, 1793–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stotesbury, H.; Kirkham, F.J.; Koelbel, M.; Balfour, P.; Clayden, J.D.; Sahota, S.; Sakaria, S.; Saunders, D.E.; Howard, J.; Kesse-Adu, R.; et al. White matter integrity and processing speed in sickle cell anemia. Neurology 2018, 90, e2042–e2050. [Google Scholar] [CrossRef] [PubMed]
- Katz, T.; Schatz, J.; Roberts, C.W. Comorbid obstructive sleep apnea and increased risk for sickle cell disease morbidity. Sleep Breath. 2018, 22, 797–804. [Google Scholar] [CrossRef]
- Bills, S.E.; Katz, T.; McNeil, J.; Schatz, J. Does Obstructive Sleep Apnea Increase Cognitive Deficits in Pediatric Sickle Cell Disease? J. Int. Neuropsychol. Soc. 2019, 25, 922–930. [Google Scholar] [CrossRef]
- Rogers, V.E.; Lewin, D.S.; Winnie, G.B.; Geiger-Brown, J. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J. Clin. Sleep Med. 2010, 6, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Samuels, M.P.; Stebbens, V.A.; Davies, S.C.; Picton-Jones, E.; Southall, D.P. Sleep related upper airway obstruction and hypoxaemia in sickle cell disease. Arch. Dis. Child. 1992, 67, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Efird, J.T.; Knupp, C.; Kadali, R.; Liles, D.; Shiue, K.; Boettger, P.; Quan, S.F. Sleep Disorders in Adult Sickle Cell Patients. J. Clin. Sleep Med. 2015, 11, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Wittig, R.M.; Roth, T.; Keenum, A.J.; Sarnaik, S. Snoring, Daytime Sleepiness, and Sickle Cell Anemia. Am. J. Dis. Child 1988, 142, 589. [Google Scholar] [CrossRef]
- Brennan, L.C.; Kirkham, F.J.; Gavlak, J.C. Sleep-disordered breathing and comorbidities: Role of the upper airway and craniofacial skeleton. Nat. Sci. Sleep 2020, 11, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.L.; DeBaun, M.R.; Strunk, R.C.; Redline, S.; Seicean, S.; Craven, D.I.; Gavlak, J.C.; Wilkey, O.; Inusa, B.; Roberts, I.; et al. Obstructive sleep apnea and sickle cell anemia. Pediatrics 2014, 134, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hankins, J.; Verevkina, N.I.; Smeltzer, M.P.; Wu, S.; Aygun, B.; Clarke, D.F. Assessment of Sleep-Related Disorders in Children With Sickle Cell Disease. Hemoglobin 2014, 38, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Wallen, G.R.; Minniti, C.P.; Krumlauf, M.; Eckes, E.J.; Allen, D.; Oguhebe, A.; Seamon, C.; Darbari, D.S.; Hildesheim, M.; Yang, L.; et al. Sleep disturbance, depression and pain in adults with sickle cell disease. BMC Psychiatry 2014, 14, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Lee, S.-A.; Ryu, H.U.; Chung, Y.-S.; Kim, W.S. Quality of life in patients with obstructive sleep apnea. Chronic Respir. Dis. 2016, 13, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Daniel, L.C.; Barakat, L.P. A Review of Sleep Concerns in Paediatric Sickle Cell Disease. Eur. Oncol. Haematol. 2012, 8, 58. [Google Scholar] [CrossRef]
- Rogers, V.E.; Marcus, M.C.L.; Jawad, A.F.; Smith-Whitley, K.; Ohene-Frempong, K.; Bowdre, C.; Allen, J.L.; Arens, R.; Mason, T.B. Periodic Limb Movements and Disrupted Sleep in Children with Sickle Cell Disease. Sleep 2011, 34, 899–908. [Google Scholar] [CrossRef]
- Lehmann, G.C.; Bell, T.R.; Kirkham, F.J.; Gavlak, J.C.; Ferguson, T.F.; Strunk, R.C.; Austin, P.; Rosen, C.L.; Marshall, M.J.; Wilkey, O.; et al. Enuresis Associated with Sleep Disordered Breathing in Children with Sickle Cell Anemia. J. Urol. 2012, 188, 1572–1577. [Google Scholar] [CrossRef]
- Valrie, C.R.; Gil, K.M.; Redding-Lallinger, R.; Daeschner, C. The Influence of Pain and Stress on Sleep in Children with Sickle Cell Disease. Child. Health Care 2007, 36, 335–353. [Google Scholar] [CrossRef]
- Blader, J.C.; Koplewicz, H.S.; Abikoff, H.; Foley, C. Sleep Problems of Elementary School Children. Arch. Pediatr. Adolesc. Med. 1997, 151, 473–480. [Google Scholar] [CrossRef]
- Johnson, E.O.; Roth, T.; Schultz, L.; Breslau, N. Epidemiology of DSM-IV Insomnia in Adolescence: Lifetime Prevalence, Chronicity, and an Emergent Gender Difference. Pediatrics 2006, 117, e247–e256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, T.D.; Lundahl, A.; Molfese, D.L.; Waford, R.N.; Roman, A.; Gozal, D.; Molfese, V.J.; Ferguson, M.C. Estimating Child Sleep From Parent Report of Time in Bed: Development and Evaluation of Adjustment Approaches. J. Pediatr. Psychol. 2014, 39, 624–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combs, D.; Goodwin, J.L.; Quan, S.F.; Morgan, W.J.; Hsu, C.-H.; Edgin, J.O.; Parthasarathy, S. Mother Knows Best? Comparing Child Report and Parent Report of Sleep Parameters with Polysomnography. J. Clin. Sleep Med. 2019, 15, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosby, B.; LeBourgeois, M.K.; Harsh, J.R. Racial Differences in Reported Napping and Nocturnal Sleep in 2- to 8-Year-Old Children. Pediatrics 2005, 115, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Hall, M.; Dahl, R.E. Sleep in Healthy Black and White Adolescents. Pediatrics 2014, 133, e1189–e1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.A.; Jackson, C.L.; Williams, N.J.; Alcántara, C. Are sleep patterns influenced by race/ethnicity—A marker of relative advantage or disadvantage? Evidence to date. Nat. Sci. Sleep 2019, 11, 79–95. [Google Scholar] [CrossRef] [Green Version]
- Kingsbury, J.H.; Buxton, O.M.; Emmons, K.M.; Redline, S. Sleep and its Relationship to Racial and Ethnic Disparities in Cardiovascular Disease. Curr. Cardiovasc. Risk Rep. 2013, 7, 387–394. [Google Scholar] [CrossRef]
- Owens, J.A.; Babcock, D.; Weiss, M. Evaluation and Treatment of Children and Adolescents with Excessive Daytime Sleepiness. Clin. Pediatr. 2020, 59, 340–351. [Google Scholar] [CrossRef]
- Joyce, A.; Hill, C.M.; Dimitriou, D.; Karmiloff-Smith, A. Sleep enhances memory consolidation in children. J. Sleep Res. 2013, 23, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Astill, R.G.; Van Der Heijden, K.B.; Van Ijzendoorn, M.H.; Van Someren, E.J.W. Sleep, cognition, and behavioral problems in school-age children: A century of research meta-analyzed. Psychol. Bull. 2012, 138, 1109–1138. [Google Scholar] [CrossRef]
- Slater, G.; Steier, J. Excessive daytime sleepiness in sleep disorders. J. Thorac. Dis. 2012, 4, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Patterson, F.; Malone, S.K.; Grandner, M.A.; Lozano, A.; Perkett, M.; Hanlon, A. Interactive effects of sleep duration and morning/evening preference on cardiovascular risk factors. Eur. J. Public Health 2018, 28, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Eid, B.; Saleh, M.B.; Melki, I.; Torbey, P.-H.; Najem, J.; Saber, M.; El Osta, N.; Khabbaz, L.R. Evaluation of Chronotype Among Children and Associations With BMI, Sleep, Anxiety, and Depression. Front. Neurol. 2020, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Ameringer, S.; Elswick, R.K.; Smith, W. Fatigue in adolescents and young adults with sickle cell disease: Biological and behavioral correlates and health-related quality of life. J. Pediatr. Oncol. Nurs. 2013, 31, 6–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameringer, S.; Smith, W.R. Emerging Biobehavioral Factors of Fatigue in Sickle Cell Disease. J. Nurs. Sch. 2011, 43, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kaleyias, J.; Mostofi, N.; Grant, M.; Coleman, C.; Luck, L.; Dampier, C.; Kothare, S.V. Severity of Obstructive Sleep Apnea in Children With Sickle Cell Disease. J. Pediatr. Hematol. 2008, 30, 659–665. [Google Scholar] [CrossRef]
- Strauss, T.; Sin, S.; Marcus, C.L.; Mason, T.B.A.; McDonough, J.M.; Allen, J.L.; Caboot, J.B.; Bowdre, C.Y.; Jawad, A.F.; Smith-Whitley, K.; et al. Upper Airway Lymphoid Tissue Size in Children With Sickle Cell Disease. Chest 2012, 142, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Valrie, C.R.; Trout, K.L.; Bond, K.E.; Ladd, R.J.; Huber, N.L.; Alston, K.J.; Sufrinko, A.; Everhart, E.; Fuh, B.R. Sleep Problem Risk for Adolescents With Sickle Cell Disease. J. Pediatr. Hematol. 2018, 40, 116–121. [Google Scholar] [CrossRef]
- Mullin, J.E.; Cooper, B.P.; Kirkham, F.J.; Rosen, C.L.; Strunk, R.C.; DeBaun, M.R.; Redline, S.; Kemp, J.S. Stability of Polysomnography for One Year and Longer in Children with Sickle Cell Disease. J. Clin. Sleep Med. 2012, 8, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Willen, M.S.; Cohen, R.; Rodeghier, M.; Kirkham, F.; Redline, S.S.; Rosen, C.; Kirkby, J.; DeBaun, M.R. Age is a predictor of a small decrease in lung function in children with sickle cell anemia Shaina. Am. J. Hematol. 2018, 93, 408–415. [Google Scholar] [CrossRef]
- Hollocks, M.J.; Kok, T.B.; Kirkham, F.J.; Gavlak, J.; Inusa, B.P.; DeBaun, M.R.; De Haan, M. Nocturnal Oxygen Desaturation and Disordered Sleep as a Potential Factor in Executive Dysfunction in Sickle Cell Anemia. J. Int. Neuropsychol. Soc. 2011, 18, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.C.; Kirkham, F.J.; Redline, S.; Rosen, C.L.; Yan, Y.; Roberts, I.; Gruenwald, J.; Marek, J.; DeBaun, M.R. Left ventricular hypertrophy and diastolic dysfunction in children with sickle cell disease are related to asleep and waking oxygen desaturation. Blood 2010, 116, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric Properties of a Survey Instrument for School-Aged Children. Sleep 2000, 23, 1–9. [Google Scholar] [CrossRef]
- Downes, M. The Development and Assessment of Executive Functioning in Preschool Children with and without Sickle Cell Anaemia. Ph.D. Thesis, UCL (University College London), London, UK, 2016. [Google Scholar]
- Thomas, M.S.; Annaz, D.; Ansari, D.; Scerif, G.; Jarrold, C.; Karmiloff-Smith, A.; Dimitriou, D. Using Developmental Trajectories to Understand Developmental Disorders. J. Speech Lang. Hear. Res. 2009, 52, 336–358. [Google Scholar] [CrossRef] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.A.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-Analysis of Quantitative Sleep Parameters from Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values across the Human Lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Biggs, S.N.; Lushington, K.; Martin, A.J.; Heuvel, C.V.D.; Kennedy, J.D. Gender, socioeconomic, and ethnic differences in sleep patterns in school-aged children. Sleep Med. 2013, 14, 1304–1309. [Google Scholar] [CrossRef]
- Markovich, A.N.; Gendron, M.A.; Corkum, P.V. Validating the Children’s Sleep Habits Questionnaire against Polysomnography and Actigraphy in School-Aged Children. Front. Psychiatry 2015, 5, 188. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.; Li, Y.; Rueschman, M.N.; Winkelman, J.W.; Ellenbogen, J.M.; Solet, J.M.; Dulin, H.; Berkman, L.F.; Buxton, O.M. Measuring Sleep: Accuracy, Sensitivity, and Specificity of Wrist Actigraphy Compared to Polysomnography. Sleep 2013, 36, 1747–1755. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Tarokh, L. Developmental changes in sleep biology and potential effects on adolescent behavior and caffeine use. Nutr. Rev. 2014, 72, 60–64. [Google Scholar] [CrossRef]
- Reddy, K.R.B.K.; Lim, M.T.C.; Lee, T.J.; Goh, D.Y.T.; Ramamurthy, M.B. Pediatric polysomnographic studies at a tertiary-care hospital in Singapore. Indian Pediatr. 2014, 51, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.; Lehman, E.; Hicks, S.; Novick, M.B. Bedtime Use of Technology and Associated Sleep Problems in Children. Glob. Pediatr. Health 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.; Jowett, S. Sleep problems in pre-school children: A review of the literature. Child Care Health Dev. 1994, 20, 379–391. [Google Scholar] [CrossRef]
- Costa, A.; Barros, H.; Santos, A. Sleep onset delay and night awakenings in preschool children: The Generation XXI birth cohort. Sleep Med. 2013, 14, e41. [Google Scholar] [CrossRef]
- Nixon, G.M.; Thompson, J.M.D.; Han, D.Y.; Becroft, D.M.O.; Clark, P.M.; Robinson, E.; Waldie, K.E.; Wild, C.J.; Black, P.N.; A Mitchell, E. Falling asleep: The determinants of sleep latency. Arch. Dis. Child. 2009, 94, 686–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceglie, G.; Di Mauro, M.; De Jacobis, I.T.; De Gennaro, F.; Quaranta, M.; Baronci, C.; Villani, A.; Palumbo, G. Gender-Related Differences in Sickle Cell Disease in a Pediatric Cohort: A Single-Center Retrospective Study. Front. Mol. Biosci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, A.M.; Fok, T.F.; Wing, Y.K. Roles of Parental Sleep/Wake Patterns, Socioeconomic Status, and Daytime Activities in the Sleep/Wake Patterns of Children. J. Pediatr. 2010, 156, 606–612.e5. [Google Scholar] [CrossRef]
- Patrick, K.E.; Millet, G.; Mindell, J.A. Sleep Differences by Race in Preschool Children: The Roles of Parenting Behaviors and Socioeconomic Status. Behav. Sleep Med. 2015, 14, 467–479. [Google Scholar] [CrossRef]
- Wolf, R.B.; Kassim, A.A.; Goodpaster, R.L.; DeBaun, M.R. Nocturnal enuresis in sickle cell disease. Expert Rev. Hematol. 2014, 7, 245–254. [Google Scholar] [CrossRef]
- Downes, M.; De Haan, M.; Kirkham, F.J.; Telfer, P.T. Parent reported sleep problems in preschool children with sickle cell anemia and controls in East London. Pediatr. Blood Cancer 2016, 64, e26337. [Google Scholar] [CrossRef]
- Maslowsky, J.; Ozer, E.J. Developmental Trends in Sleep Duration in Adolescence and Young Adulthood: Evidence from a National United States Sample. J. Adolesc. Heal. 2014, 54, 691–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, J.; Jean-Louis, G.; Zizi, F.; Casimir, G.J.; Von Gizycki, H.; Brown, C.D.; McFarlane, S.I. Sleep Duration among Black and White Americans: Results of the National Health Interview Survey. J. Natl. Med Assoc. 2008, 100, 317–322. [Google Scholar] [CrossRef]
- Gladwin, M.T. Cardiovascular complications in patients with sickle cell disease. Hematology 2017, 2017, 423–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarlane, I.M.; Ozeri, D.; Saperstein, Y.; Alvarez, M.R.; Leon, S.Z.; Koci, K.; Francis, S.; Singh, S.; Salifu, M. Rheumatoid Arthritis in Sickle-Cell Population: Pathophysiologic Insights, Clinical Evaluation and Management. Rheumatol. Curr. Res. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Koelbel, M.; Brenchley, C.; Laurence, A.; Sahota, S.; Kirkham, F.; Dimitriou, D. Do you remember? Sleep fragmentation and immediate memory recall in sickle cell anaemia. Sleep Med. 2019, 64, S201. [Google Scholar] [CrossRef]
- Traeger, N.; Schultz, B.; Pollock, A.N.; Mason, T.; Marcus, C.L.; Arens, R. Polysomnographic values in children 2–9 years old: Additional data and review of the literature. Pediatr. Pulmonol. 2005, 40, 22–30. [Google Scholar] [CrossRef]
- Anderson, L.M.; Allen, T.M.; Thornburg, C.D.; Bonner, M.J. Fatigue in children with sickle cell disease: Association with neurocognitive and social-emotional functioning and quality of life. J. Pediatr. Hematol. 2015, 37, 584–589. [Google Scholar] [CrossRef]
- Owens, J.; Adolescent Sleep Working Group; Committee on Adolescence; Au, R.; Carskadon, M.; Millman, R.; Wolfson, A.; Braverman, P.K.; Adelman, W.P.; Breuner, C.C.; et al. Insufficient Sleep in Adolescents and Young Adults: An Update on Causes and Consequences. Pediatrics 2014, 134, e921–e932. [Google Scholar] [CrossRef] [Green Version]
- Tarokh, L.; Saletin, J.M.; Carskadon, M.A. Sleep in adolescence: Physiology, cognition and mental health. Neurosci. Biobehav. Rev. 2016, 70, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, A.; Martin, S.; Wolters, P.; Rodriguez, Y.; Toledo-Tamula, M.A.; Struemph, K.; Fitzhugh, C.; Hsieh, M.; Tisdale, J. Sleep disturbance in adults with sickle cell disease: Relationships with executive and psychological functioning. Ann. Hematol. 2020, 99, 2057–2064. [Google Scholar] [CrossRef]
- Baughn, J.M.; Lechner, H.G.; Herold, D.L.; Brown, V.A.; Moore, W.R.; Harris, C.D.; Stehr, H.I.; Sorensen, C.M.; Cleveland, E.J.; Akason, J.D.; et al. Enhancing the patient and family experience during pediatric sleep studies. J. Clin. Sleep Med. 2020, 16, 1037–1043. [Google Scholar] [CrossRef]
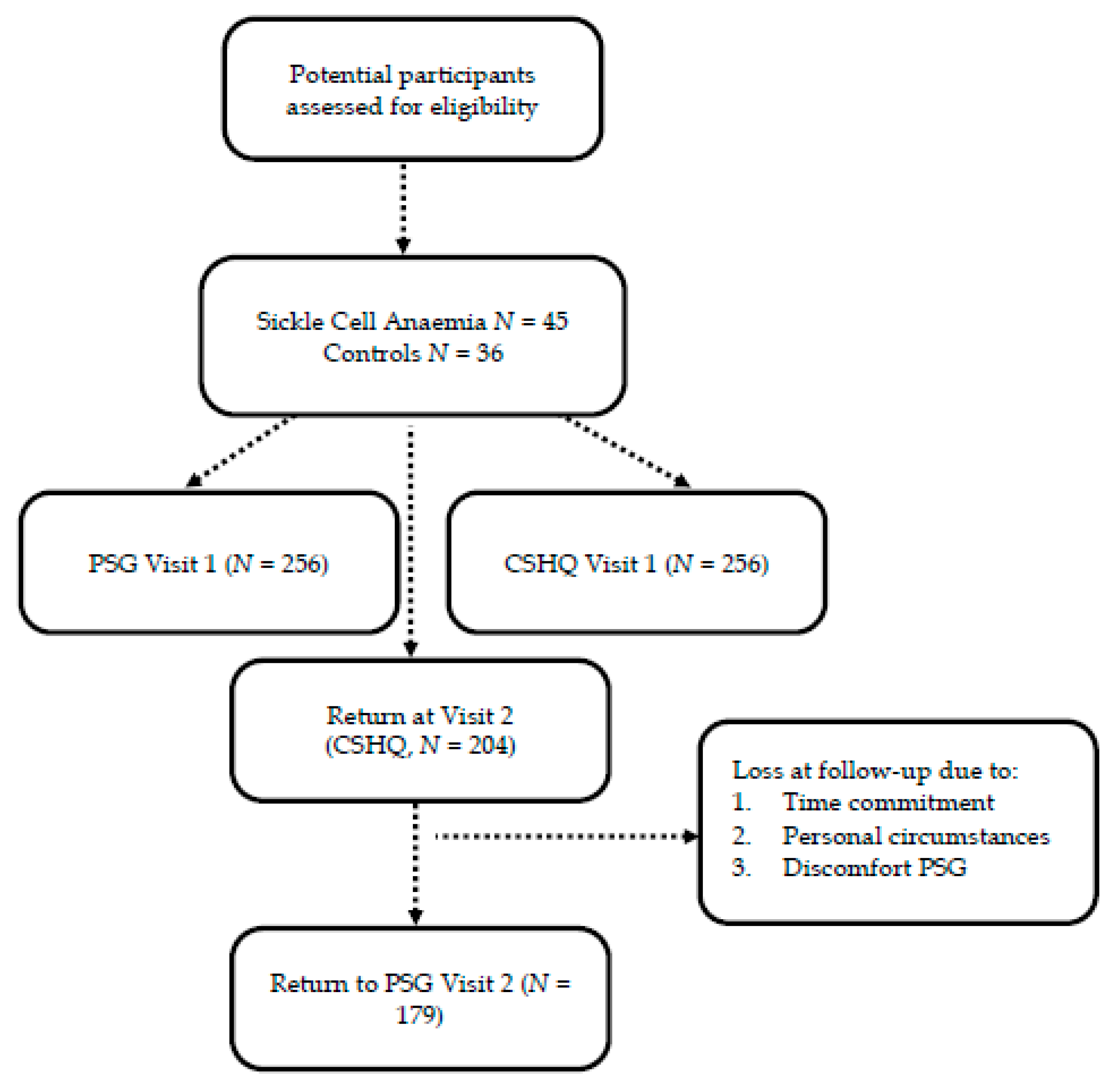
| Visit 1 | Visit 2 | No 2nd Visit | V1-V2 | V2-No 2nd Visit | |
|---|---|---|---|---|---|
| (N), % or Mean ± SD | p | ||||
| Age in years | 11 ± 4.5 (255) | 14.9 ± 4.4 (179) | 14.9 ± 5.2 (26) | p < 0.001 | p > 0.05 |
| Gender male | 51% | 50% | 58% | p > 0.05 | p > 0.05 |
| Hemoglobin | 8.3 ± 1.3 (252) | N/A | N/A | N/A | N/A |
| Hydroxyurea | 12.5% (241) | 14.4% (167) | 11% (8) | p > 0.05 | p > 0.05 |
| Genotype | (257) | (179) | (77) | p > 0.05 | p < 0.001 |
| SS | 90.3% | 95.60% | 68.8% | ||
| Beta 0 Thalassemia | 3.9% | 2% | 13.0% | ||
| SC | 5.4% | 2.4% | 18.2% | ||
| Black Heritage | (257) | (179) | (78) | p > 0.05 | p > 0.05 |
| UK | 36.2% | 36.0% | 37% | ||
| USA | 63.8% | 64.0% | 63% | ||
| Primary Caregiver | (257) | (179) | (78) | p > 0.05 | p > 0.05 |
| Mother | 83.7% | 86.0% | 78% | ||
| Father | 7% | 5.6% | 10% | ||
| Other | 9.3% | 8.4% | 11% | ||
| Marital Status | (257) | (179) | (78) | p < 0.001 | p < 0.001 |
| Married | 15.2% | 11.7% | 23.0% | ||
| Single | 36.2% | 31.3% | 47.4% | ||
| Living with Partner | 5.1% | 3.9% | 7.7% | ||
| Divorced/Separated | 35% | 44.7% | 12.8% | ||
| SES: Income | (257) | (154) | (64) | p > 0.05 | p > 0.05 |
| Upper middle class | 3.9% | 3.90% | 6% | ||
| Middle class | 16.3% | 19.50% | 19% | ||
| Low income | 33.5% | 37.00% | 39% | ||
| Near poverty level | 31.1% | 37.00% | 36% | ||
| SES: Education | (257) | (176) | (76) | p > 0.05 | p > 0.05 |
| Academic Degree | 19.8% | 17.6% | 26.3% | ||
| Highschool/College | 65.8% | 70.0% | 60.5% | ||
| Primary/Secondary | 12.5% | 12.5% | 13.0% | ||
| Sleep PSG | (256) | (179) | (77) | ||
| AHI | 2.5 ± 4.7 | 2.7 ± 4.5 | 2 ± 5.2 | p = 0.021 | p > 0.05 |
| OAHI | 2 ± 4.5 | 1.5 ± 2.7 | 1.5 ± 4.5 | p > 0.05 | p > 0.05 |
| O2 | 95.7 ± 3.6 | 95 ± 3.8 | 96.5 ± 2.9 | p = 0.015 | p = 0.001 |
| TST PSG | 7 h 13 min | 6 h 34 min | 6 h 53 min | p < 0.001 | p = 0.043 |
| N | CSHQ V1 | CSHQ V2 | CSHQ V2 | N | PSG V1 | PSG V2 | PSG V2 | N | CSHQ V1 | N | CSHQ V2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSHQ V1 | PSG V1 | PSG V1 | PSG V2 | |||||||||
| △ Mean ± SD | △ Mean ± SD | △ Mean ± SD | △ Mean ± SD | |||||||||
| Week+ Weekend | ||||||||||||
| Bedtime | 196 | 21:55 (01:13) | 22:51 (01:27) | 00:56 (01:17) ** | 170 | 22:11 (02:01) | 22:24 (00:45) | 00:13 (02:01) ** | 248 | –00:15 (01:47) * | 170 | 00:27 (01:27) * |
| 19:00–03:00 | 20:00–03:30 | 20:05–21:51 | 20:06–00:22 | |||||||||
| Sleep Latency | 195 | 36.7 (30.0) | 39.8 (33.1) | 3.1 (38.9) | 170 | 32.9 (36.4) | 26.5 (31.9) | −6.4 (47.1) * | 248 | 5.91 (46.0) * | 169 | 14.2 (43.2) ** |
| 0–180 | 0–180 | 0–235 | 0–181 | |||||||||
| Waketime | 197 | 08:05 (00:57) | 08:33 (1:23) | 00:28 (01:16) ** | 170 | 06:57 (00:37) | 06:29 (0:22) | –00:28 (00:42) ** | 250 | 01:09 (01:09) ** | 170 | 02:04 (01:30) ** |
| 05:45–11:00 | 05:45–14:00 | 02:44–08:17 | 4:59–07:18 | |||||||||
| Hours per day | 197 | 09:23 (01:189 | 08:47 (01:26) | –00:36 (01:38) ** | 170 | 07:20 (01:05) | 06:34 (01:11) | –00:46 (01:25) ** | 249 | 02:07 (01:36) ** | 170 | 02:11 (01:40)** |
| 04:00–15:00 | 03:00–13:30 | 03:42–10:02 | 01:46–08: 49 | |||||||||
| Week | ||||||||||||
| Bedtime | 200 | 21:19 (01:13) | 22:04 (01:25) | 00:45 (01:18) ** | 50 | 22:14 (01:00) | 22:37 (00:50) | 00:23 (01:01) * | 80 | –01:08 (01:22) ** | 101 | –00:17 (01:33) * |
| 19:00–03:00 | 19:30–03:00 | 20:50–01:11 | 20:06–00:22 | |||||||||
| Sleep Latency | 198 | 39.4 (32.7) | 40.7 (37.92) | 1.3 (42.1) | 50 | 27.5 (21.9) | 32.1 (35.7) | 4.6 (43.1) | 80 | 6.9 (44.0) * | 101 | 15.6 (49.0) * |
| 0–180 | 0–180 | 0–117 | 0–150 | |||||||||
| Waketime | 199 | 06:59 (00:53) | 07:18 (01:26) | 00:18 (01:32) | 50 | 06:47 (00:21) | 06:27 (00:25) | –00:20 (00:34) ** | 80 | 00:23 (00:55) * | 101 | 01:00 (01:35) ** |
| 04:00–11:00 | 05:00–15:00 | 05:3–07:38 | 04:59–07:14 | |||||||||
| Hours per day | 199 | 08:46 (01:29) | 08:13 (01:33) | –00:33 (01:48) ** | 50 | 7:03 (00:58) | 06:12 (01:14) | –00:51 (01:01) ** | 80 | 02:12 (01:41) ** | 101 | 01:48 (01:41) ** |
| 04:00–11:49 | 03:10–13:00 | 04:49–08:55 | 02:10–08:19 | |||||||||
| Weekend | ||||||||||||
| Bedtime | 197 | 22:32 (01:28) | 23:39 (01:46) | 01:06 (01:45) ** | 120 | 22:09 (02:19) | 22:19(00:42) | 00:09 (02:18) * | 170 | 00:29 (02:08) ** | 69 | 01:44 (01:54) ** |
| 19:00–04:00 | 20:00–05:00 | 20:05–21:51 | 20:18–00:16 | |||||||||
| Sleep Latency | 197 | 33.7 (35.3) | 38.4 (39.5) | 4.7 (49.3) | 120 | 35.1 (40.9) | 24.1 (30.0) | –11.0 (48.1) * | 170 | 7.0 (55.0) | 69 | –7.1 (43.6) |
| 0–180 | 0–240 | 0–235 | 0–181 | |||||||||
| Waketime | 200 | 09:12 (01:35) | 09:50 (02:00) | 00:38 (01:51) ** | 120 | 07:01 (00:41) | 06:30 (00:21) | –00:31 (00:44) ** | 174 | 02:11 (01:38) ** | 69 | 03:18 (02:13) ** |
| 05:30–14:00 | 05:00–15:00 | 02:44–08:17 | 05:29–07:18 | |||||||||
| Hours per day | 198 | 10:00 (01:41) | 09:21 (02:01) | –00:38 (02:19) ** | 120 | 07:27 (01:07) | 06:43 (01:08) | –00:43 (01:21) ** | 171 | 02:25 (01:50) ** | 66 | 03:03 (01:55) ** |
| 04:00–19:00 | 00:30–16:00 | 03:42–10:02 | 01:46–08:49 |
| N | PSG Visit 1 | PSG Visit 2 | PSG V2-PSG V1 | p | |
|---|---|---|---|---|---|
| △ Mean (±SD) | |||||
| AHI | 170 | 2.7 (4.5) | 1.9 (2.8) | 0.8 (4.2) | p = 0.021 |
| 0–37.4 | 0–19.6 | ||||
| WASO | 170 | 59.1 (41.9) | 63.7 (53.2) | −4.7 (62.5) | p > 0.05 |
| 9–277.5 | 5.5–378 | ||||
| Night waking | 170 | 25.4 (8.2) | 26.4 (9.6) | −1 (10.8) | p > 0.05 |
| 8–74 | 8–61 | ||||
| PLM | 169 | 5.9 (10.5) | 4.7 (8.2) | 1.2 (9.8) | p = 0.002 |
| 0–117.3 | 0–72.7 | ||||
| O2 Saturation | 170 | 95.3 (3.8) | 94.9 (3.8) | 0.4 (2.8) | p = 0.015 |
| 81.2–100 | 80.5–100 |
| N | CSHQ Visit 1 | CSHQ Visit 2 | CSHQ V2-CSHQ V1 | p | |
|---|---|---|---|---|---|
| △ Mean (±SD) | |||||
| CSHQ CS | 200 | 14.9 (10.1) | 13.5 (9.7) | 1.4 (10.7) | p = 0.037 |
| 0–49 | 0–55 | ||||
| SDB | 200 | 3.0 (3.1) | 3.1 (3.0) | −0.02 (3.4) | p > 0.05 |
| 0–16 | 0–16 | ||||
| Parasomnias | 200 | 5.4 (4.3) | 4.6 (3.8) | 0.8 (4.3) | p = 0.019 |
| 0–21 | 0–23 | ||||
| Night wake | 200 | 3.5 (3.5) | 2.7 (3.2) | 0.7 (4.2) | p = 0.01 |
| 0–12 | 0–12 | ||||
| Bedtime resistance | 200 | 1.2 (1.5) | 1.0 (1.5) | 0.2 (1.8) | p > 0.05 |
| 0–4 | 0–4 |
| Daytime Sleepiness | ||||||
|---|---|---|---|---|---|---|
| N (CSHQ Score Mean ± SD) | Never | Not Often | Sometimes | Often | Always | p |
| CSHQ CS Visit 1 | 162 (12.2 ± 8.6) | 35 (16.1 ± 9.9) | 38 (18.1 ± 11.3) | 10 (23.3 ± 7.5) | 10 (25.4 ± 9.8) | p < 0.001 |
| CSHQ CS Visit 2 | 102 (9.1 ± 6.6) | 23 (12.5 ± 5.8) | 47 (16.7 ± 8.8) | 14 (23.2 ± 13.6) | 15 (27.1 ± 9.2) | p < 0.001 |
| SDB Visit 1 | 162 (2.5 ± 2.8) | 35 (3.1 ± 3.1) | 38 (4 ± 3.5) | 10 (6.1 ± 3.2) | 10 (4.6 ± 3.1) | p < 0.001 |
| SDB Visit 2 | 102 (2.4 ± 2.4) | 23 (2.6 ± 1.7) | 47 (3.6 ± 3.5) | 14 (5 ± 3.9) | 15 (4.7 ± 3.9) | p < 0.05 |
| Movement at night Visit 1 | 162 (0.6 ± 1.3) | 35 (1.4 ± 1.7) | 38 (1.7 ± 2) | 10 (2.4 ± 1.8) | 10 (1.5 ± 1.8) | p < 0.001 |
| Movement at night Visit 2 | 102 (0.5 ± 1.2) | 23 (0.7 ± 1.3) | 47 (1.4 ± 1.7) | 14 (2.1 ± 2.7) | 15 (2.7 ± 2.4) | p < 0.001 |
| Night wake Visit 1 | 162 (3 ± 3.3) | 35 (3.3 ± 3.1) | 38 (3.4 ± 3.1) | 10 (3 ± 3.2) | 10 (7.3 ± 4.5) | p < 0.001 |
| Night wake Visit 2 | 102 (1.7 ± 2.6) | 23 (2.7 ± 2.7) | 47 (3.7 ± 2.9) | 14 (3.9 ± 4.1) | 15 (5.9 ± 4.8) | p < 0.001 |
| Parasomnias Visit 1 | 162 (4.4 ± 4) | 35 (5.7 ± 4.4) | 38 (6.4 ± 4.4) | 10 (8.5 ± 4) | 10 (8.9 ± 5.9) | p < 0.05 |
| Parasomnias Visit 2 | 102 (3.2 ± 3.2) | 23 (4.1 ± 2) | 47 (5.6 ± 3.6) | 14 (8 ± 4.8) | 15 (9.5 ± 4.6) | p < 0.001 |
| Napping Visit 1 | 161 (0.8 ± 1.2) | 35 (1.2 ± 1.3) | 38 (1.2 ± 1.3) | 10 (1.8 ± 1.5) | 10 (2.4 ± 1.8) | p < 0.05 |
| Napping Visit 2 | 103 (0.9 ± 1.3) | 23 (1 ± 1.1) | 48 (1.7 ± 1.4) | 14 (2.5 ± 1) | 15 (1.5 ± 1.8) | p < 0.001 |
| TST Visit 1 | 159 (9:18 ± 1:21) | 35 (9:11 ± 1:18) | 38 (9:31 ± 1:04) | 10 (9:23 ± 1:23) | 8 (9:59 ± 1:17) | p > 0.05 |
| TST Visit 2 | 100 (8:49 ± 1:23) | 23 (8:44 ± 1:09) | 48 (8:50 ± 1:33) | 14 (8:58 ± 1:27) | 15 (8:13 ± 2:03) | p > 0.05 |
| Haemoglobin Visit 1 | 159 (8.3 ± 1.4) | 34 (8.4 ± 1) | 38 (8.4 ± 1.4) | 10 (8.4 ± 1) | 9 (7.9 ± 1.5) | p > 0.05 |
 | ||||||
| Total Sleep Time | ||||
|---|---|---|---|---|
| Model | Variable | β | p-Value | R2 |
| Step 1 | Age | −0.17 | 0.007 | 0.03 * |
| Step 2 | Age | −0.19 | 0.003 | 0.06 * |
| O2 | 0.06 | 0.365 | ||
| PLMI | −0.16 | 0.013 | ||
| Step 3 | Age | −0.23 | <0.001 | 0.54 ** |
| O2 | −0.14 | 0.002 | ||
| PLMI | −0.11 | 0.027 | ||
| SOL | 0.3 | <0.001 | ||
| WASO | −0.48 | <0.001 | ||
| Night waking | 0.16 | 0.001 | ||
| Waketime | 0.41 | <0.001 | ||
| Total Sleep Time | ||||
|---|---|---|---|---|
| Model | Variable | β | p-Value | R2 |
| Step 1 | Age | −0.46 | <0.001 | 0.22 ** |
| Female | 0.05 | 0.349 | ||
| Step 2 | Age | −0.45 | <0.001 | 0.25 ** |
| Female | 0.06 | 0.319 | ||
| Hydroxyurea | −0.11 | 0.058 | ||
| AHI | −0.03 | 0.682 | ||
| O2 | −0.03 | 0.694 | ||
| PLMI | 0.15 | 0.012 | ||
| Step 3 | Age | −0.43 | <0.001 | 0.27 ** |
| Female | 0.07 | 0.258 | ||
| Hydroxyurea | −0.12 | 0.042 | ||
| AHI | −0.03 | 0.589 | ||
| O2 | −0.03 | 0.603 | ||
| PLMI | 0.15 | 0.009 | ||
| SES | 0.13 | 0.024 | ||
| Step 4 | Age | −0.49 | <0.001 | 0.55 ** |
| Female | 0.04 | 0.403 | ||
| Hydroxyurea | −0.09 | 0.048 | ||
| AHI | −0.06 | 0.229 | ||
| O2 | −0.07 | 0.182 | ||
| PLMI | 0.12 | 0.007 | ||
| SES | 0.11 | 0.022 | ||
| SOL | −0.35 | <0.001 | ||
| Waketime | 0.45 | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kölbel, M.; Kirkham, F.J.; Dimitriou, D. Developmental Profile of Sleep and Its Potential Impact on Daytime Functioning from Childhood to Adulthood in Sickle Cell Anaemia. Brain Sci. 2020, 10, 981. https://doi.org/10.3390/brainsci10120981
Kölbel M, Kirkham FJ, Dimitriou D. Developmental Profile of Sleep and Its Potential Impact on Daytime Functioning from Childhood to Adulthood in Sickle Cell Anaemia. Brain Sciences. 2020; 10(12):981. https://doi.org/10.3390/brainsci10120981
Chicago/Turabian StyleKölbel, Melanie, Fenella J. Kirkham, and Dagmara Dimitriou. 2020. "Developmental Profile of Sleep and Its Potential Impact on Daytime Functioning from Childhood to Adulthood in Sickle Cell Anaemia" Brain Sciences 10, no. 12: 981. https://doi.org/10.3390/brainsci10120981
APA StyleKölbel, M., Kirkham, F. J., & Dimitriou, D. (2020). Developmental Profile of Sleep and Its Potential Impact on Daytime Functioning from Childhood to Adulthood in Sickle Cell Anaemia. Brain Sciences, 10(12), 981. https://doi.org/10.3390/brainsci10120981